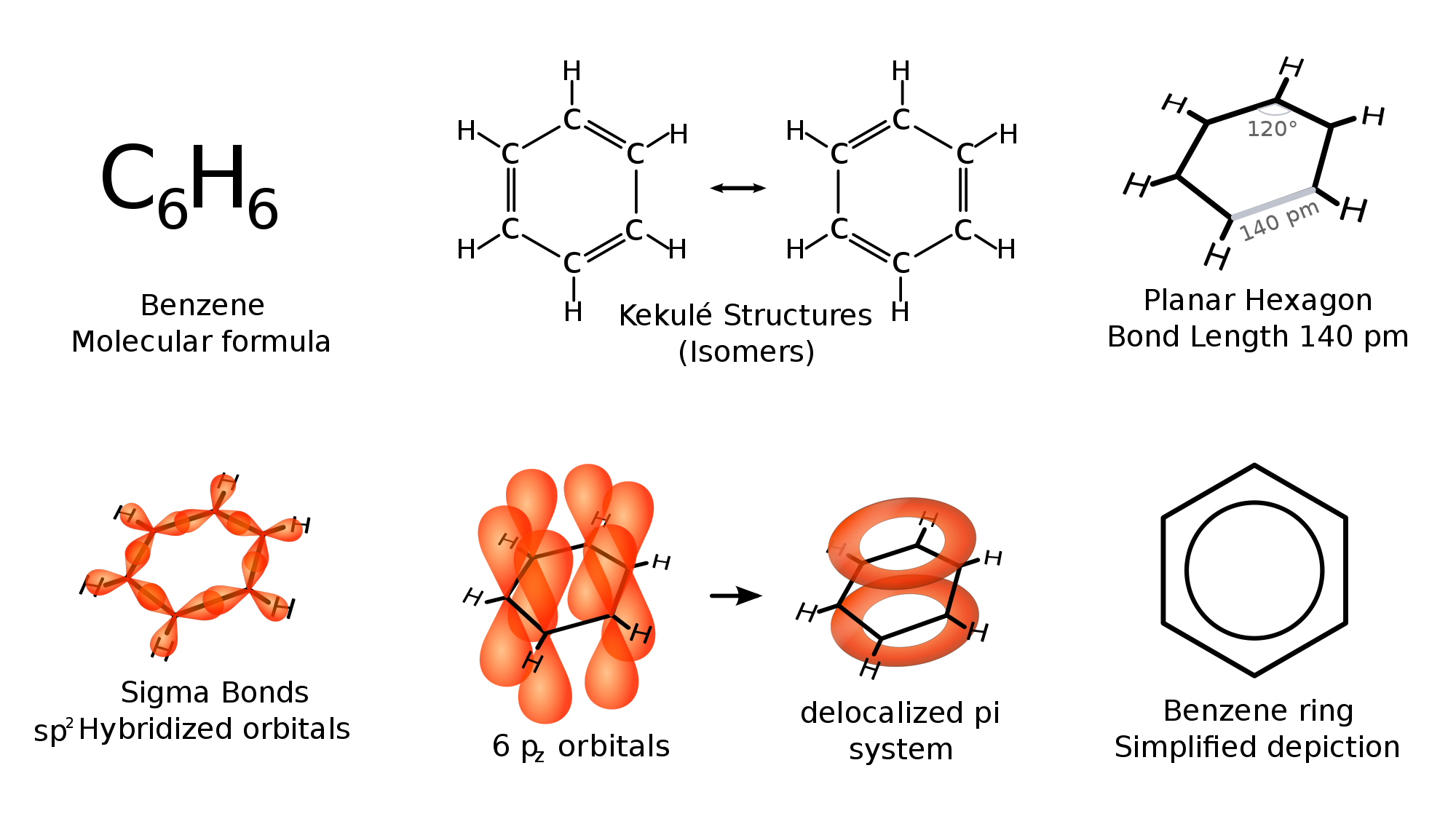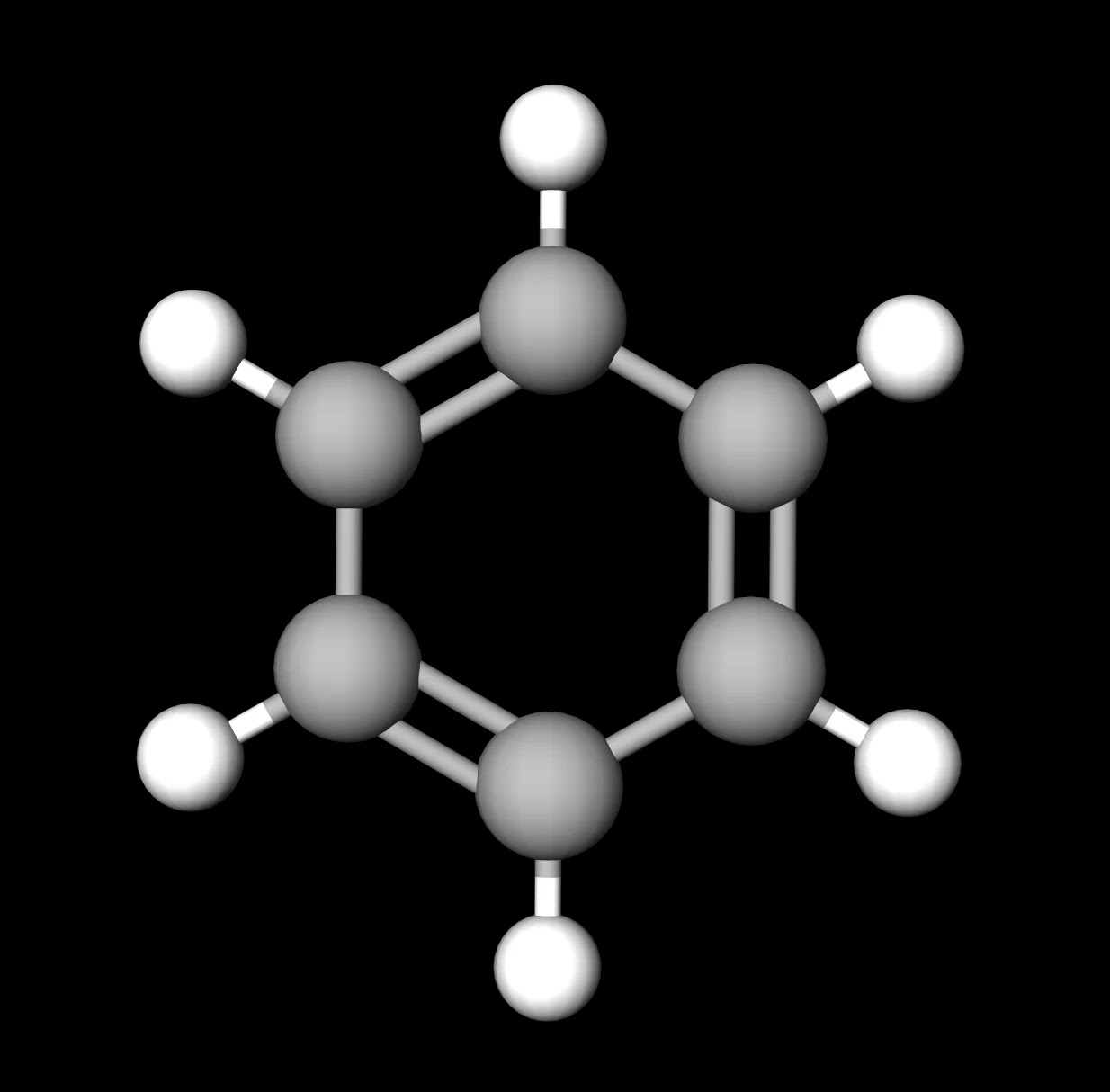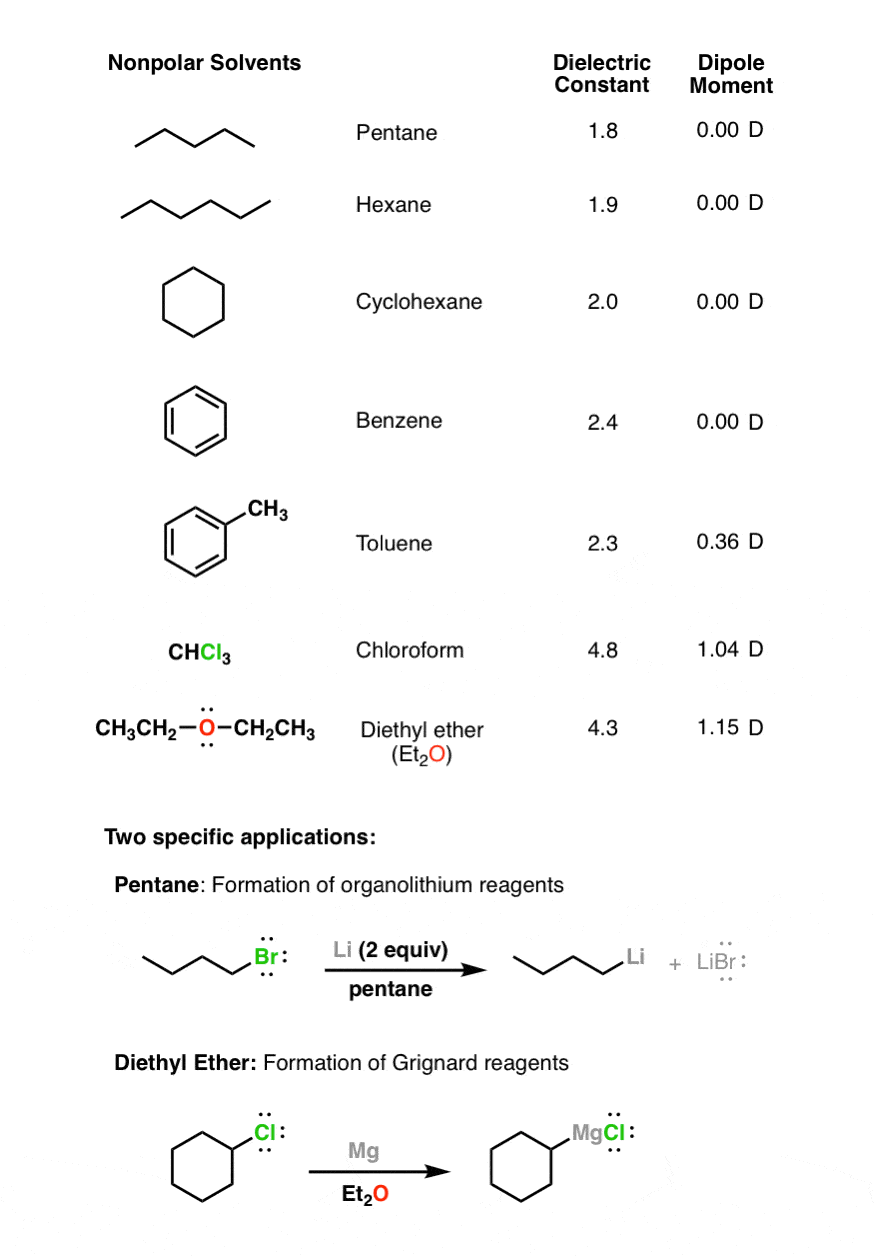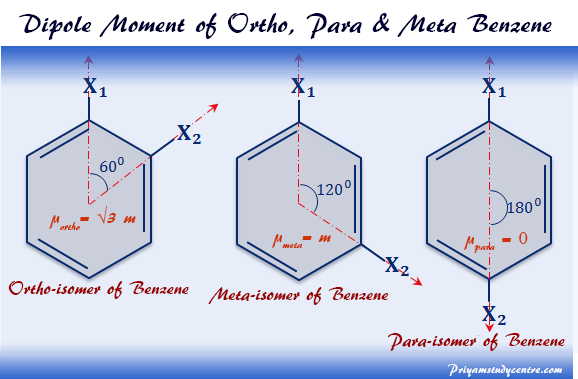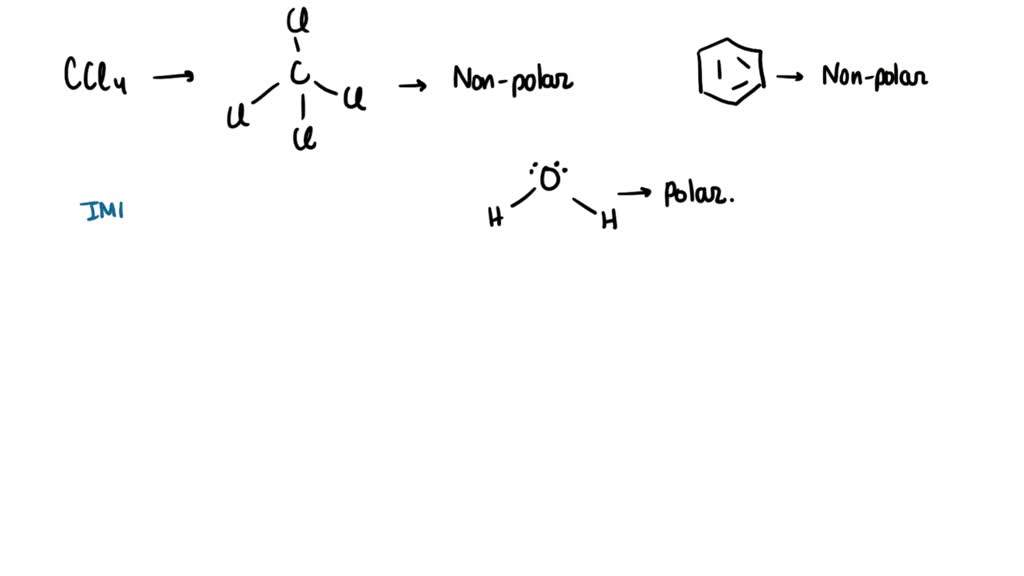
SOLVED: Carbon tetrachloride, CCI4(l), is more mixable with benzene, C6H6(l), than with H2O because (1) the intermolecular forces are similar in both carbon tetrachloride and benzene. (2) both carbon tetrachloride and benzene
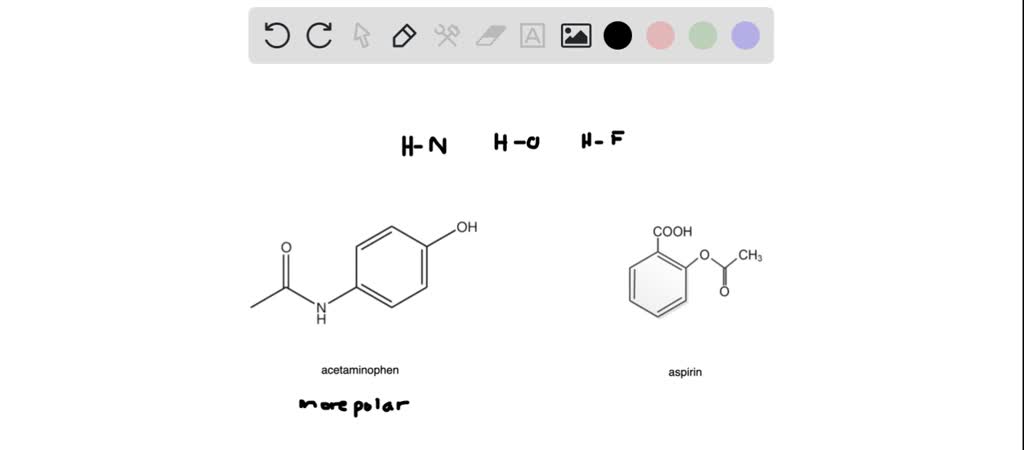
SOLVED: Aspirin has a non polar benzene ring, a polar carboxyl group and a polar ester group. Phenacetin has a non polar benzene ring, a non polar ether and a polar amide

SOLVED:Water is a polar solvent and benzene is a nonpolar solvent. In which solvent is each of the following solutes more likely to be soluble? a. hexane b. gasoline c. KCl d.

text{ }{{\\text{C}}_{\\text{6}}}{{\\text{H}}_{\\text{6}}}\\text{ }$ is a very good industrial solvent for:A) $\\text{ NaCl }$B) $\\text{ MgC}{{\\text{l}}_{\\text{2}}}\\text{ }$C) $\\text{ CaC}{{\\text{O}}_{\\text{3}}}\\text{ }$D) Fats